There is still much to be learned about the potential roles of septin structures and structural biology can contribute a lot to this!
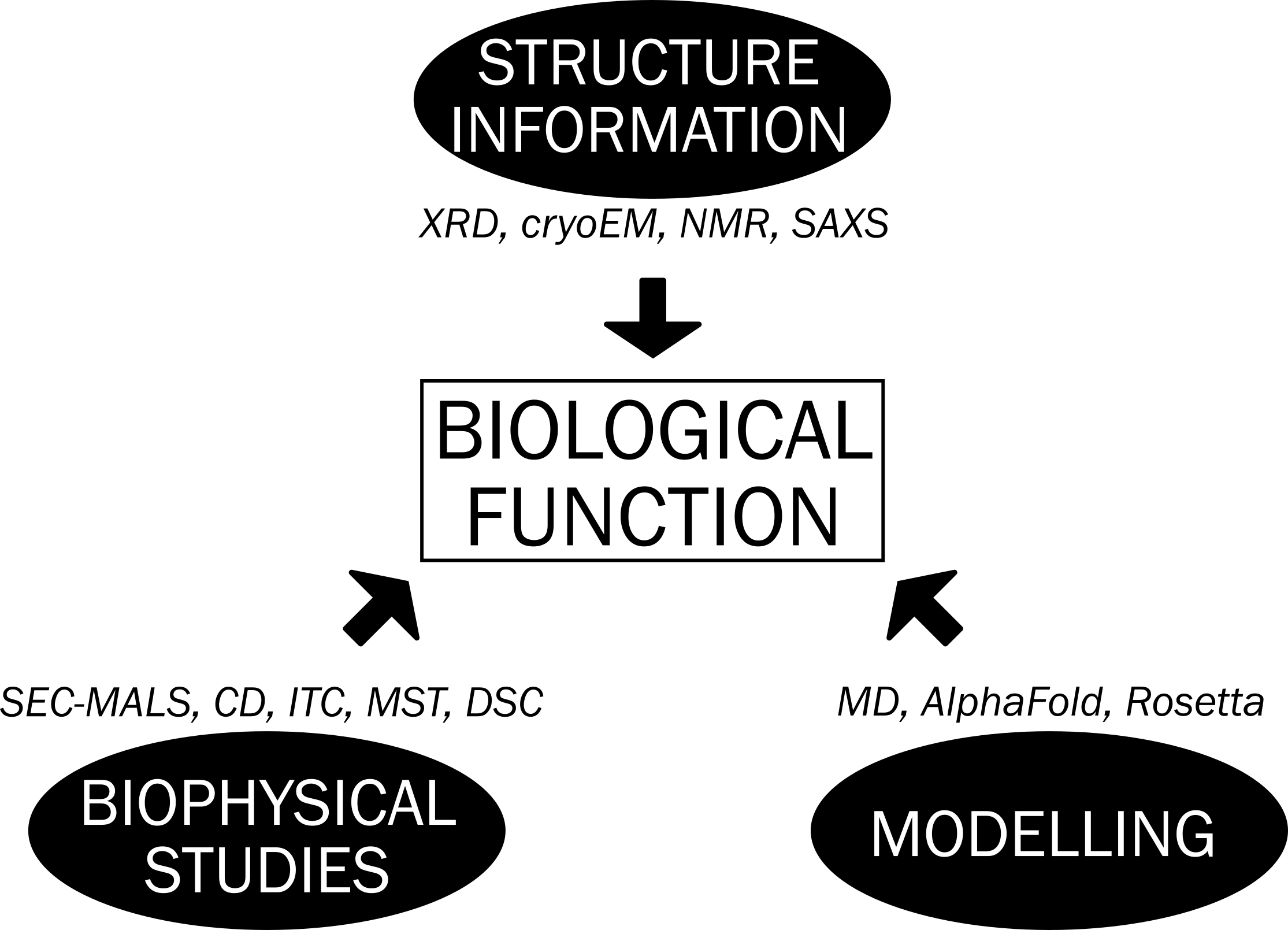
We use an integrative approach to understand how septins assemble and, ultimately, their biological relevance.
Below, we describe our current research goals, divided into three major themes:
Understanding the mechanism of oligomerization and dynamics of septin complexes
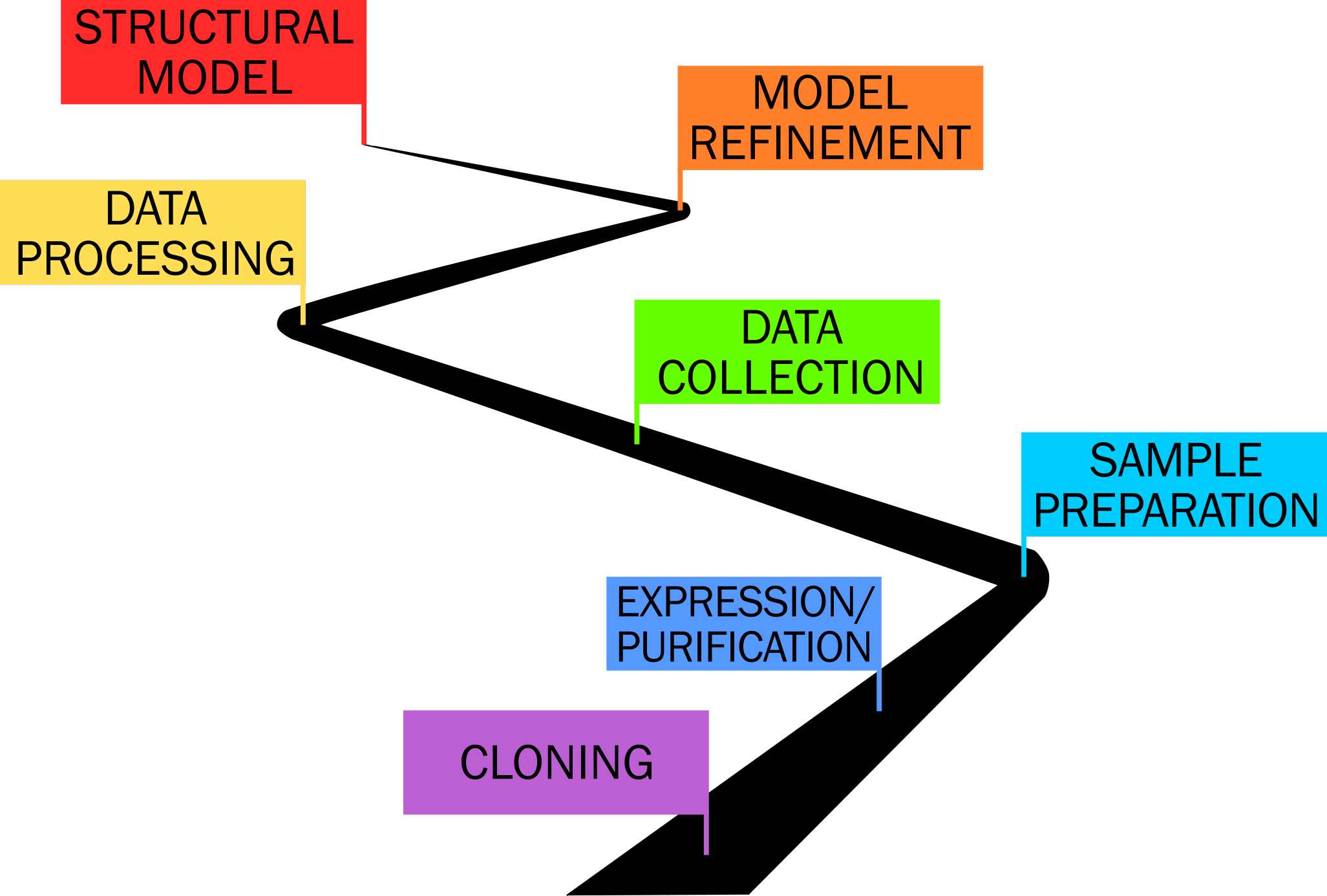
The arduous road towards the elucidation of a protein structure!
- Better understand the flexibility oligomers/filaments have and how those correlate with the septin ability in recognizing membrane curvature.
Flexibility modes of SEPT2-6-7 oligomers revealed by cryo-EM (Mendonça et al. 2021, reproduced with permission)
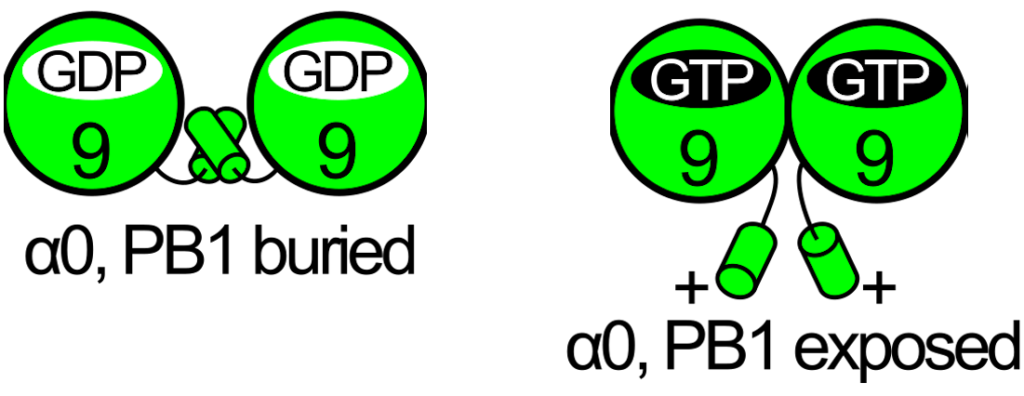
- Test the hypothesis of the opening of the NC-interface between two copies of septins of this group when complexed to GDP (Castro et al. 2020). If correct, then it should be possible to detect a difference in octamer length in the two cases.
- Investigate the formation of a rare covalent bond (Cys-Lys, Asn-Lys) between septin subunits (Rosa et al. 2020).
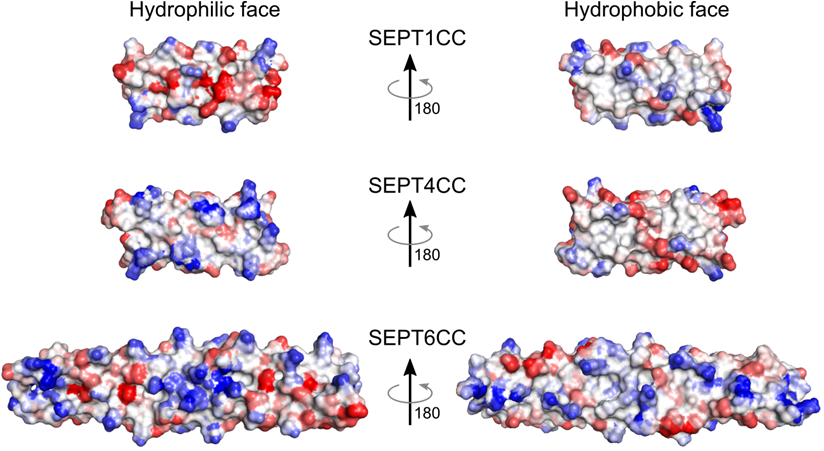
- Understand the NC interface between SEPT6 and SEPT7 and its peculiar heteromeric coiled-coil (Leonardo et al. 2021).
- Unravel the structural biology of septins from non-human species where there is currently a dearth of structural information.
Septin engineering
- Assess the importance of the C-terminal domain in the selection of partners for septin-septin interaction using coiled-coil chimeras.
- Study septin interfaces through non-canonical oligomers composed of a septin mutant (interrupted filaments).
- Insert catalytic activity to a non-active septin. It is much harder to introduce/build biological activity compared to simply eliminating it.
Interaction with non-septin partners
A huge breakthrough in the septin field will happen once we better comprehend how septins interact with non-septin components and how those regulate the septin cytoskeleton. We just began to characterize these challenging complexes and impressive results are emerging!