The Structural Biology of Septins website
Our group is hosted at the Physics Institute of São Carlos (IFSC) at the University of São Paulo (USP), São Carlos, Brazil. Over the course of the last ten years, we have accumulated considerable experience in the production of recombinant human septins and their complexes and have grown to be the largest group in the world working on the septin structure (see a bit of our history).
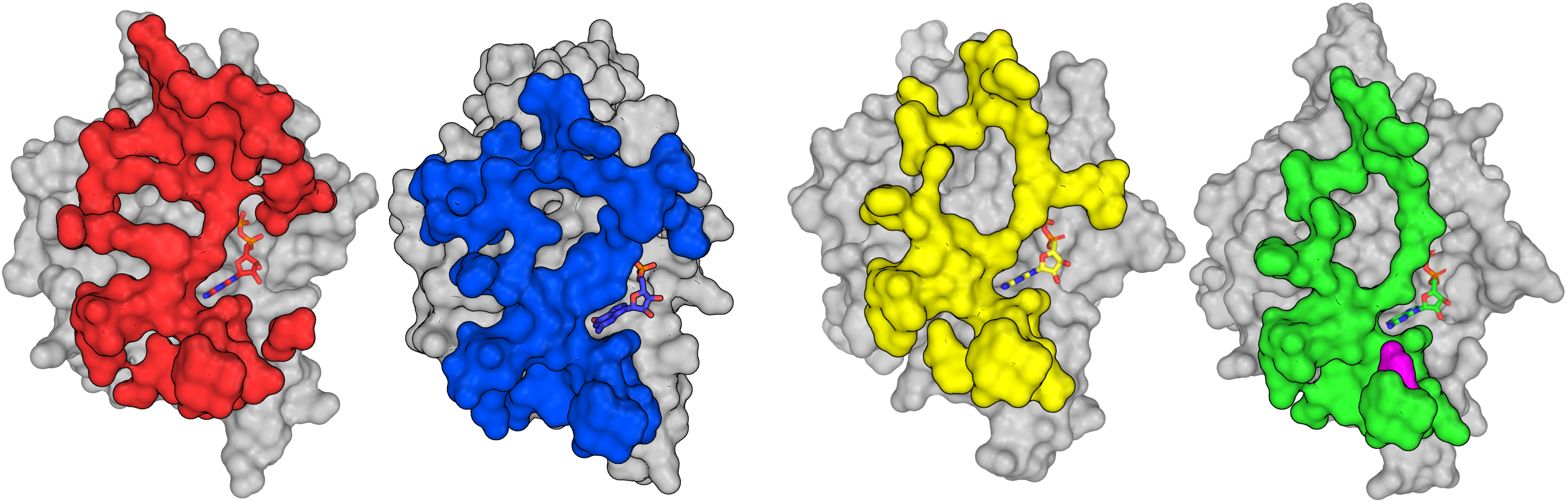
We have solved several human septin structures, in isolation or in complex (see our Structural Gallery). Our main interest is in the structural aspect of septins and in the molecular recognition processes they employ to form heterofilaments. See the page Research for more information.
What are septins?
Septins are guanine nucleotide-binding proteins and have the ability to form highly organized filaments. They were discovered 50 years ago, forming a collar at the budding neck (septum) in yeast cells during division. Since then, septins have been associated with several important processes that demand membrane changes and/or scaffold structures.
In humans, thirteen genes (sept1-12 and sept14) code for different protein products which are divided into four septin groups based on sequence homology (SEPT2, SEPT6, SEPT7 and SEPT3; we usually refer to them by using the red, blue, yellow and green colors, respectively).
Three or four human septins hetero-oligomerize, forming hexamers or octamers. Septin subunits use two distinct interfaces to heteropolymerize: the G-interface, formed by the central G-domain and the NC-interface, composed of the terminal N- and C-domains. It is believed that septins from the same group are interchangeable, i.e., one can replace another in the same position (see more in Kinoshita’s rule).
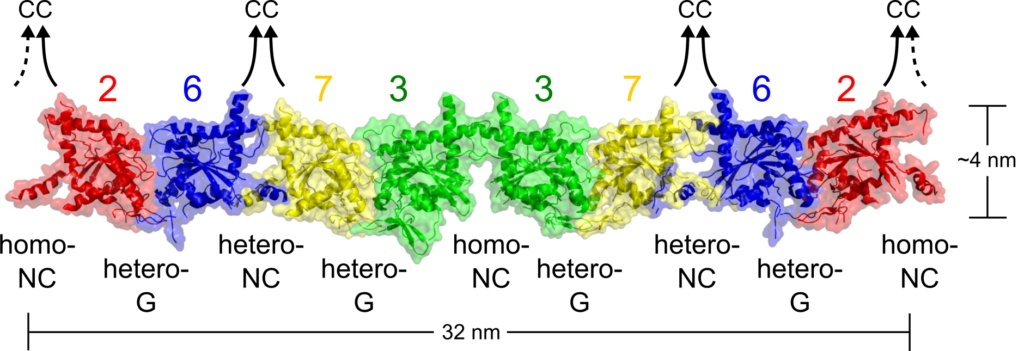
Representation of a human septin octamer.
Share this page with other septin aficionados!